The Fascinating Science of Snowflake Formation and Uniqueness
Written on
Chapter 1: The Enchantment of Snowfall
In the UK, snowfall is a rarity, which makes each occurrence a delightful event. When the first flakes drift down, a sense of wonder sweeps over the town. As the snow begins to settle, children and parents alike eagerly peer outside, asking, “Will it stick?” Often, the snow melts away almost as swiftly as it arrives, leaving only a glossy sheen on the roads.
However, there are magical moments when the snow accumulates, transforming the landscape into a pristine white blanket. Last winter, I woke my husband at 6:30 AM to experience the beauty of freshly fallen snow. The darkness required us to use headlamps, and as we stepped outside, our familiar surroundings appeared softened, as if an artist had blurred the sharp edges of reality.
Early mornings in winter are typically quiet, but the stillness of a snow-covered world takes on a profound quality. It feels almost sacred, as if I am wandering through an enormous, icy cathedral. And the splendor of snowflakes themselves is captivating. Have you ever examined one closely? Their intricate designs are breathtaking.
How are these enchanting structures created? Is it true that no two snowflakes are exactly the same?

Photo by Mona Hamm on Unsplash
Snowflakes originate in the chilly, moisture-laden clouds high above us. The formation requires a delicate balance of high humidity and low temperatures. In extremely cold conditions (below -35°C), frozen water droplets can adhere to one another. In slightly warmer conditions (above -35°C), droplets can freeze around particles like dust or pollen, known as a ‘nucleus’. As more frozen water collects on this initial structure, ice crystals begin to form.
Why are snowflakes characterized by their six-sided shape? Depending on the environmental conditions, they can develop into various structures, including prism-like or needle-shaped forms, in addition to the well-known branched plate shapes that many adore.
Photo by Brian0918 — Credit: Erbe, Pooley: USDA, ARS, EMU, Public Domain.
All these structures share a common trait: six-sided symmetry. This phenomenon is linked to how water molecules arrange themselves during ice formation. The polar nature of water molecules plays a significant role in this process. In a water molecule, the oxygen atom is slightly more negatively charged than the hydrogen atoms. Thus, when they cluster together to freeze, they tend to arrange themselves in specific patterns.
Imagine scattering bar magnets on a table; they align based on their magnetic properties. Water molecules behave similarly, leading to the beautiful hexagonal structure depicted below:
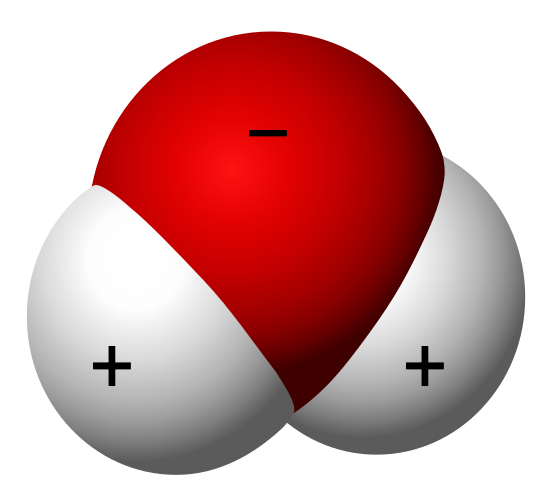
By File:Water molecule 3D.svg:derivative work: MikeRun — This file was derived from: Water molecule 3D.svg:, Public Domain.
As additional water molecules attach to the snowflake, the hexagonal framework continues to expand.
Are all snowflakes truly unique? If water molecules arrange in a specific manner during ice formation, why don't all snowflakes resemble flat hexagons? The intricate details emerge as snowflakes grow over time. Each flake descends through varying atmospheric conditions, altering its shape and branching. For example, when the air is highly saturated, larger, more branched snowflakes can develop. Scientists categorize snowflakes based on their common shapes.
The final snowflake design mirrors the diverse conditions encountered during its atmospheric journey. A sudden change in branching signifies a shift in environmental factors, and thanks to molecular symmetry, these transformations appear on all six sides of the snowflake.
The outcome? A distinctive structure that appeals to our appreciation for symmetry.

Photo by Marc Newberry on Unsplash
Due to the vast array of environmental conditions a growing snowflake may experience, it is nearly impossible to find two identical naturally formed snowflakes. However, in controlled laboratory environments, researchers have managed to create snowflakes with identical appearances. Kenneth Libbrecht from the California Institute of Technology refers to these as ‘identical twin snowflakes’. Watching these snowflakes form can be incredibly soothing.
Even in these lab-grown ‘identical twins’, atomic variations still exist. This is attributed to the natural presence of isotopes of hydrogen in water molecules, leading to random distribution throughout each snowflake.

Photo by Ali Inay on Unsplash
The poetic nature of snowflake formation speaks volumes about their journey through our ever-changing atmosphere. Despite the randomness of their creation, symmetry emerges — a narrative of order within chaos.
Now, I'm off to admire images of snowflakes while enjoying a steaming cup of hot chocolate. This might just become my new favorite way to unwind (literally).
Key source: Kenneth G. Libbrecht, 2005, "The Physics of Snow Crystals," Rep. Prog. Phys. 68 855.
Chapter 2: Exploring Snowflake Uniqueness
The first video, "Why is every snowflake unique?", delves into the science behind the individuality of snowflakes, explaining how their unique formations arise from varying atmospheric conditions.
The second video, "How Do Snowflakes Form?", offers an in-depth look at the process of snowflake creation, revealing the intricate chemistry involved in their formation.